![SOLVED: Calculate Ka and pKa of the acid using pH and molarity.moles unknown acid = 0.001215 molar mass of acid = 172.84molarity = 0.243 mol/LpH= 2.06kA= [A-][H3O+] / [HA]please include a rice SOLVED: Calculate Ka and pKa of the acid using pH and molarity.moles unknown acid = 0.001215 molar mass of acid = 172.84molarity = 0.243 mol/LpH= 2.06kA= [A-][H3O+] / [HA]please include a rice](https://cdn.numerade.com/ask_previews/f6745ba3-6b77-4e7a-9de2-7d980958d194_large.jpg)
SOLVED: Calculate Ka and pKa of the acid using pH and molarity.moles unknown acid = 0.001215 molar mass of acid = 172.84molarity = 0.243 mol/LpH= 2.06kA= [A-][H3O+] / [HA]please include a rice
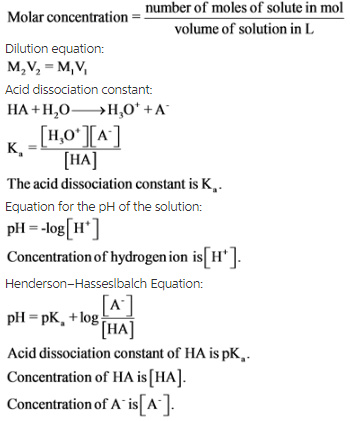
The Ka value for acetic acid, CH3COOH(aq), is 1.8x10^-5. Calculate the ph of a 2.80 M acetic acid solution - Home Work Help - Learn CBSE Forum

A 0.21 M solution of chloroacetic acid, ClCH2CO2H, has a pH of 1.79. Calculate Ka for the acid. | Homework.Study.com
![Calculate Ka of acetic acid from equilibrium concentration given below: [H(3)O^(+)]=[CH(3)COO^(-)]=1.34xx10^(-3)M, [CH(3)COOH]=9.866xx10^(-2)M Calculate Ka of acetic acid from equilibrium concentration given below: [H(3)O^(+)]=[CH(3)COO^(-)]=1.34xx10^(-3)M, [CH(3)COOH]=9.866xx10^(-2)M](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/161343719_web.png)


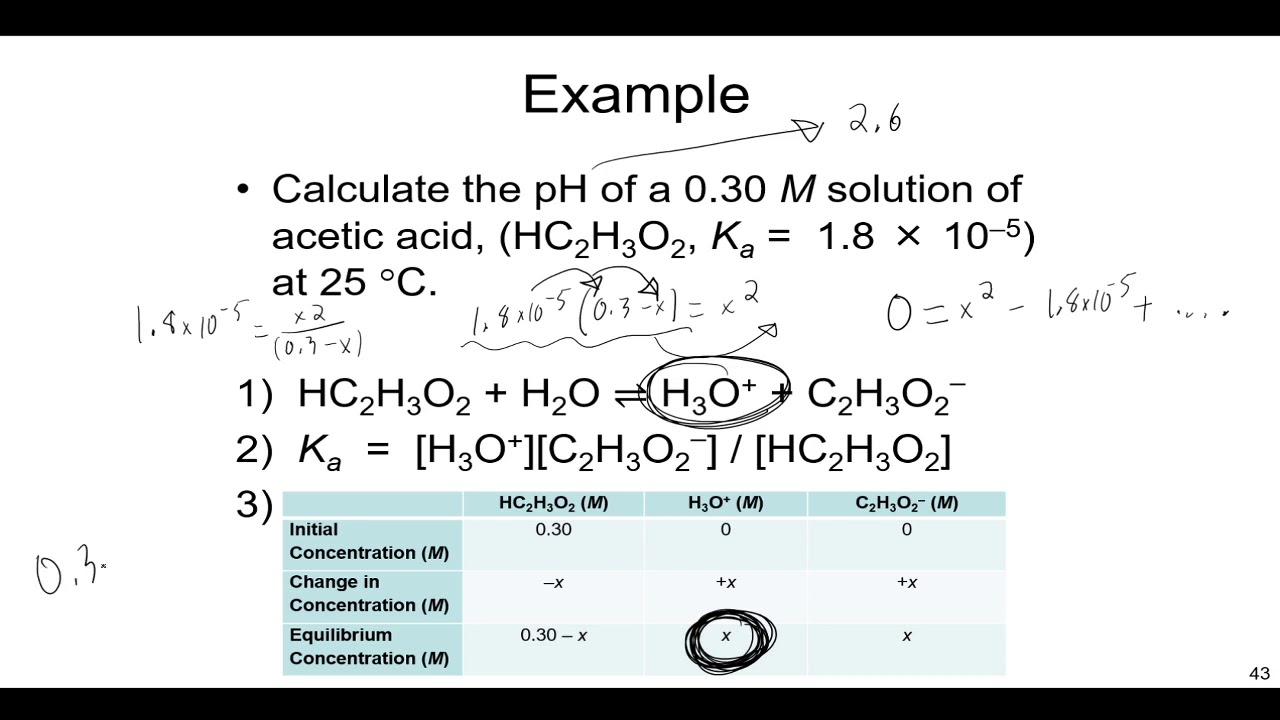


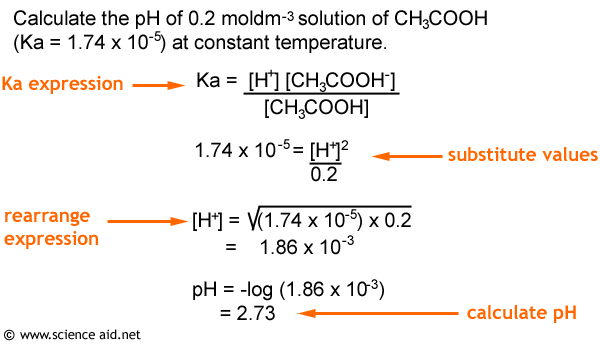
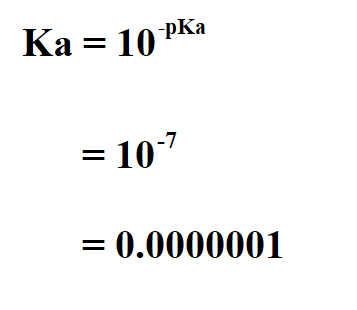



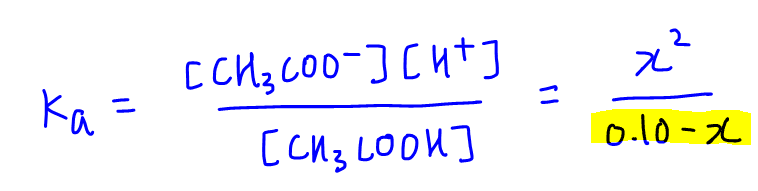

![Solved 3. Calculate the [H3O+], [OH-], pH and pOH of a 0.120 | Chegg.com Solved 3. Calculate the [H3O+], [OH-], pH and pOH of a 0.120 | Chegg.com](https://media.cheggcdn.com/study/f5c/f5c5a593-319b-4d2b-9ea3-2e5d84e0d782/image)
![Calculating [H+] and pH from Ka Calculating [H+] and pH from Ka](https://www.mi.mun.ca/users/pfisher/chemistry1011_134/img013.gif)